Introduction
Short Bowel Syndrome (SBS) is a rare and severe malabsorption disorder resulting from the surgical removal of a significant portion of the small intestine. Short Bowel Syndrome Pipeline Drugs Market This condition leads to malnutrition and severe nutrient deficiencies, making effective treatment essential for patient quality of life. The pharmaceutical industry is actively developing new drugs to treat SBS, focusing on enhancing nutrient absorption and improving gut function. This article provides a comprehensive analysis of the drugs currently in development for short bowel syndrome, exploring the latest market trends, growth drivers, and future outlook for the SBS treatment market.
Overview of Short Bowel Syndrome (SBS)
SBS often occurs as a consequence of extensive intestinal surgery, such as procedures for Crohn’s disease, cancer, or trauma. Patients with SBS face difficulties absorbing nutrients, electrolytes, and fluids, which can lead to severe complications and a significantly reduced quality of life. Traditional treatment options include dietary adjustments, intravenous nutrition, and medications aimed at slowing intestinal transit time. However, these solutions often only provide temporary relief, emphasizing the need for more effective pharmacological therapies. Syndrome pipeline drugs market target insights, download a free report sample
Short Bowel Syndrome Drug Development Landscape
The development pipeline for SBS drugs includes several promising therapies designed to improve nutrient absorption and intestinal function. These drugs focus on addressing the underlying issues caused by SBS, such as poor absorption of fluids and nutrients, rather than just treating the symptoms.
Key Categories of SBS Drugs in Development
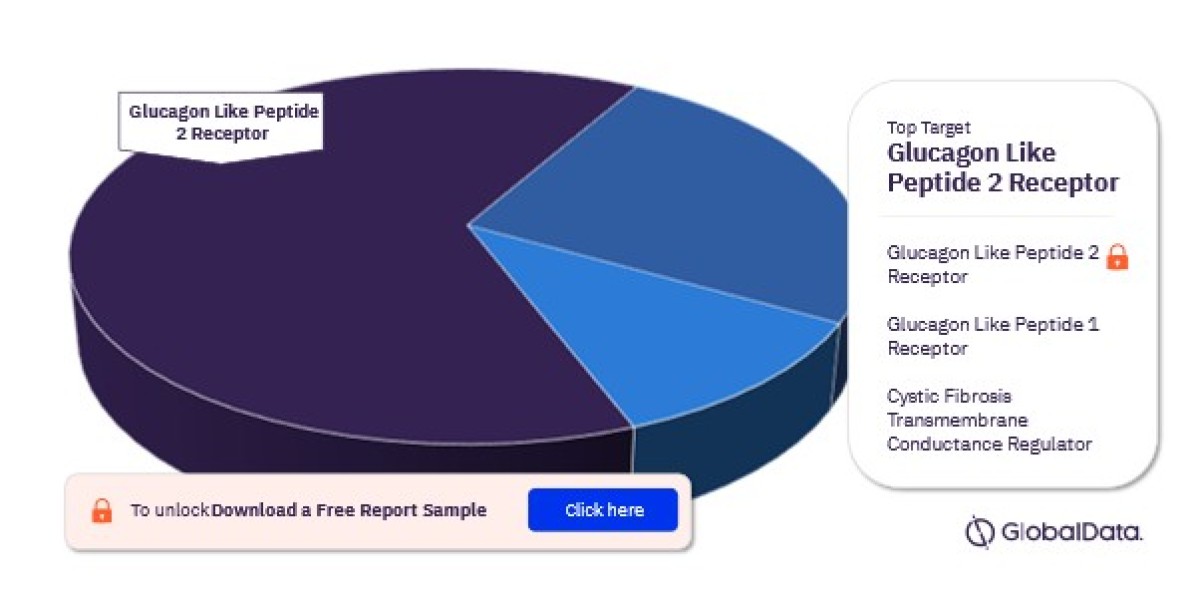
GLP-2 Analogues (Glucagon-Like Peptide-2)
- GLP-2 analogues are one of the most advanced categories of drugs in the SBS pipeline. These drugs work by enhancing intestinal growth and function, promoting nutrient absorption, and improving overall gut health. Teduglutide, a GLP-2 analogue, is an example of a successful treatment currently available in the market, and newer versions are under development to enhance efficacy and patient outcomes.
Growth Hormones and Analogues
- Growth hormone therapies are being investigated for their potential to stimulate intestinal growth and improve nutrient absorption in SBS patients. Some growth hormone analogues are currently in clinical trials, showing promising results in enhancing gut adaptation and reducing the need for parenteral nutrition.
Oral Rehydration Solutions (ORS) and Absorption Enhancers
- Drugs focusing on increasing fluid and nutrient absorption efficiency are also under development. These therapies aim to reduce dependency on intravenous nutrition by improving the body's ability to absorb necessary nutrients and fluids through the shortened intestine.
Market Drivers for SBS Drug Development
Growing Prevalence of Gastrointestinal Disorders
- The rise in gastrointestinal diseases like Crohn’s disease, cancer, and other conditions requiring surgical resection of the intestine is increasing the prevalence of SBS. This growing patient population is driving the demand for more effective drug solutions, prompting pharmaceutical companies to invest heavily in R&D for SBS treatments.
Need for Long-Term and Effective Therapies
- Traditional management strategies like parenteral nutrition are often not sustainable in the long term due to risks such as infection and liver complications. This unmet need for more effective, sustainable, and patient-friendly treatment options is a major driver for the development of new SBS drugs.
Advancements in Biopharmaceuticals
- Innovations in biopharmaceuticals, particularly biologics and peptides, are enabling the development of targeted treatments for SBS. These advanced therapies are designed to stimulate specific mechanisms within the body, promoting intestinal growth and nutrient absorption. The biopharmaceutical approach is expected to revolutionize SBS management, offering patients improved outcomes.
Challenges Facing SBS Drug Development
Complex Clinical Trials and Regulatory Approvals
- Conducting clinical trials for SBS drugs is challenging due to the rare nature of the condition and the need for long-term studies to demonstrate efficacy and safety. Regulatory approvals can be complex and require robust clinical data, making it crucial for pharmaceutical companies to navigate these hurdles effectively.
High Development Costs and Limited Patient Population
- SBS is a rare condition, and the limited patient population poses financial challenges for drug developers. The high cost of clinical trials, combined with a smaller target market, means companies must find ways to balance development costs and profitability. Partnerships, collaborations, and orphan drug designations are often strategies used to address these challenges.
Competition from Established Therapies
- While new drugs are in development, existing treatments such as parenteral nutrition and current GLP-2 analogues provide significant competition. New entrants must demonstrate superior efficacy, safety, and convenience to capture market share and convince both clinicians and patients to switch to newer therapies.
Emerging Trends in the SBS Treatment Market
Focus on Personalized Medicine
- The future of SBS treatment is expected to involve personalized medicine approaches. As understanding of the genetic and molecular aspects of SBS improves, therapies tailored to individual patients’ conditions and genetic profiles may offer more effective and targeted solutions. Personalized approaches are likely to enhance treatment outcomes, reduce side effects, and improve patient compliance.
Development of Combination Therapies
- Combination therapies that integrate multiple drug types (e.g., GLP-2 analogues combined with growth hormones or absorption enhancers) are being explored to maximize treatment efficacy. These therapies aim to address various aspects of SBS, such as enhancing gut growth while simultaneously improving absorption efficiency, thus offering a comprehensive treatment approach.
Application of Advanced Biotech Platforms
- Advanced biotech platforms, including gene editing and regenerative medicine, are being investigated for their potential in treating SBS. These cutting-edge technologies aim to repair or regenerate damaged intestinal tissues, offering new hope for patients with severe cases. While still in early research phases, these innovations may represent a breakthrough in SBS management in the future.
Future Outlook for the SBS Drug Market
The SBS drug market is expected to grow significantly as more therapies progress through clinical trials and reach commercialization. With the increasing focus on developing long-term solutions that improve patients' quality of life, pharmaceutical companies investing in SBS drug development stand to gain from this expanding market. Additionally, collaborations between biotech firms, medical research institutions, and regulatory agencies will play a crucial role in bringing innovative therapies to market faster.
Conclusion
The landscape of SBS drug development is evolving rapidly, with several promising drugs poised to enter the market. The focus on enhancing nutrient absorption, reducing reliance on parenteral nutrition, and improving patient quality of life is driving innovation in this space. Understanding the key trends, growth drivers, and challenges in SBS drug development is crucial for stakeholders seeking to capitalize on opportunities within this niche yet growing market.
Call to Action
For a comprehensive analysis of the short bowel syndrome drug development landscape, including pipeline insights, market trends, and regulatory challenges, explore GlobalData’s detailed report on Short Bowel Syndrome Drugs in Development Analysis. Gain valuable insights to make informed decisions in the SBS therapeutic market.