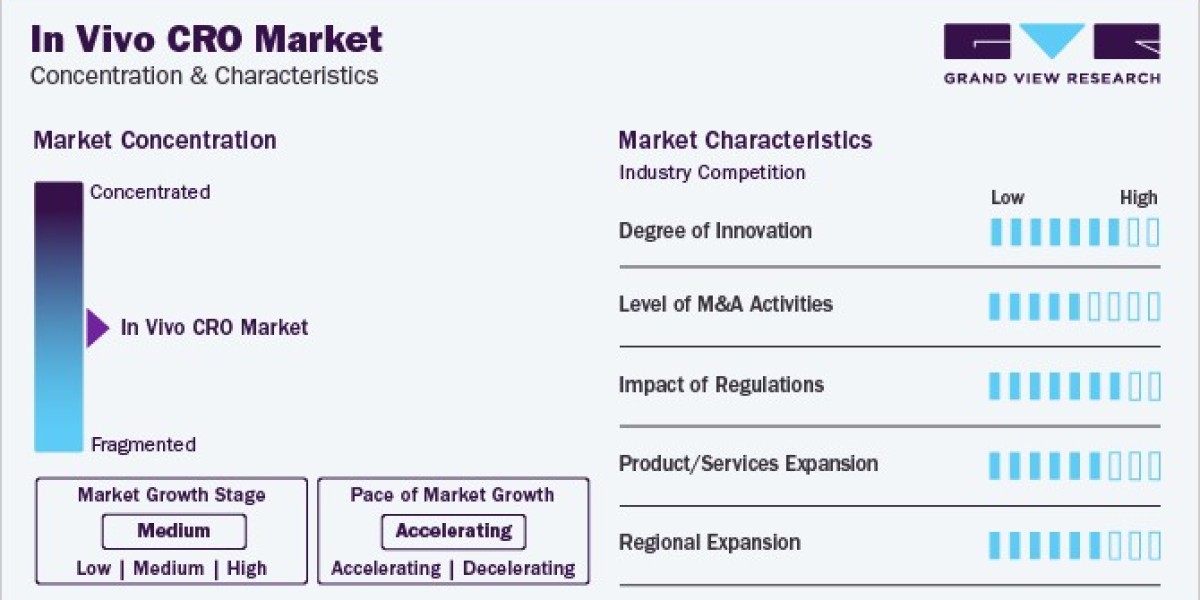
The global in vivo CRO market size was estimated at USD 4.59 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 8.1% from 2024 to 2030. The increasing burden of cancer patients, growing in vivo pharmacology studies, and the emerging number of pharma and biotechnology companies focusing on researching and developing novel therapeutics are expected to drive market growth during the forecast period.
Gather more insights about the market drivers, restrains and growth of the In vivo CRO Market
In addition, in vivo CROs are specifically backed by the rising number of CROs, research and development activities, and preclinical studies, which have led to a rise in market growth. Furthermore, growing investments across in vivo gene-modified cell therapies are expected to boost market growth. For instance, in November 2023, the CCR researchers announced the development of a new technology to support T-cell therapies to function better against solid tumors utilizing mice models with pediatric and adult cancers.
The COVID-19 pandemic did not positively impact the clinical trials and in vivo CRO industries. However, the effect of the pandemic was comparatively moderate across the in vivo CRO industry, as several contract research organizations continuously focused on the R&D of COVID-19 treatment therapeutics, thereby providing in vivo preclinical services for the same. The integrity of over 330,000 clinical trials registered on clinicaltrials.gov remains vulnerable as the coronavirus outbreak spreads worldwide. Furthermore, as of March 2020, at least 18 pharmaceutical or biotechnology companies have reported a disturbance to a clinical trial due to this pandemic. However, post-pandemic, there was a growing demand for novel technologies and an increasing need for patient-friendly drugs, which has led to improvement in the pipeline of pharmaceuticals due to stringent regulations by regulatory agencies such as the FDA and EMA, pharmaceutical & biopharmaceutical companies outsourcing their medical affairs to streamline the regulatory process. Thus, this is expected to impact market growth positively in the coming years.
Moreover, rising complexity with respect to new drug development has fueled the market’s growth. Manufacturing high-quality and safe drugs for patient care is a major concern for drug manufacturers. Moreover, the regulations related to drugs and biologics are vast and are complicated by legal technicalities as it is a rapidly growing field. The complexity of the drug development process has considerably grown in recent years. Pharmaceutical and biotechnology companies are outsourcing their complex in vivo activities to CROs and focusing on business development.
In addition, the rising demand for advanced products, such as rare disease therapies and anti-cancer medicines, is one of the major factors supporting the growth of the market. For instance, organizations such as the European Society of Medical Oncology (ESMO) and Latin American Society of Clinical Oncology (SLACOM) engage in collaborative work with the Peruvian Cooperative Oncology Group. These favorable initiatives are anticipated to generate lucrative revenue over the forecast period. Furthermore, in October 2023, Coeptis Therapeutics Holdings, Inc. announced research demonstrating the possibility of the SNAP-CAR T-cell platform to target numerous antigens. The research involved SNAP-CAR to demonstrate the technology's adaptable antigen-targeting abilities in vivo and in-vitro in xenograft models of human tumors.
Furthermore, a growing pipeline of cell and gene therapies is anticipated to increase the opportunity for market growth. Advancements in manufacturing, including improved accuracy, manufacturing, and control regulations, have led to tremendous growth in the past few years. In November 2023, the National Cancer Institute (NC) researchers designed a way to potentially improve the efficacy of T cell-based immunotherapy therapies, such as CAR T-cell therapy, to treat solid tumors. In animal-based studies, improved T-cell therapies efficiently treated neuroblastoma and cervical cancer. This is expected to increase the demand of the market, thereby contributing to market profits.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global neuromodulation devices market size was valued at USD 5.31 billion in 2023 and is projected to grow at a CAGR of 10.2% from 2024 to 2030. The rising government funding for R&D activities in these devices, the increasing prevalence of neurological disorders, and technological advancements are driving the market growth.
• The global subdermal contraceptive implants market size was valued at USD 1.05 billion in 2023 and is projected to grow at a CAGR of 7.5% from 2024 to 2030. The driving factors of the growth include rising demand for reliable contraceptives, unintended pregnancies, and increasing awareness of sexual health and family planning.
In vivo CRO Market Segmentation
Grand View Research has segmented the global in vivo CRO market based on model type, modality, indication, GLP type, and region:
In Vivo CRO Model Type Outlook (Revenue, USD Billion, 2018 - 2030)
• Rodent based
o Rat Models
o Mice Models
o Others
• Non-Rodent based
In Vivo CRO Modality Outlook (Revenue, USD Billion, 2018 - 2030)
• Small Molecules
• Large Molecules
o Cell & Gene Therapy
o CAR T-cell therapies
o CAR-NK cell therapy
o TCR-T cell therapy
o Other (Includes- TCR-NK, CARM, and TAC-T)
o RNA Therapy
o Others
In Vivo CRO Indication Outlook (Revenue, USD Billion, 2018 - 2030)
• Oncology
o Blood cancer
o Solid tumor
o Syngeneic model
o Patient derived xenograft
o Xenograft
o Others
• CNS Conditions
o Epilepsy
o Parkinson's disease
o Huntington's disease
o Stroke
o Muscular Dystrophy
o Alzheimer’s Disease
o Traumatic brain injury
o Amyotrophic lateral sclerosis (ALS)
o Spinal Muscular Atrophy
o Muscle regeneration
o Other Neurodevelopment Disorders
• Diabetes
• Obesity
• Pain management
o Chronic pain
o Acute pain
• Autoimmune/inflammation conditions
o Rheumatoid Arthritis
o Multiple Sclerosis
o Osteoarthritis
o Irritable Bowel Syndrome
o Others
• Others
In Vivo CRO GLP Type Outlook (Revenue, USD Billion, 2018 - 2030)
• GLP Toxicology
• Non GLP
In Vivo CRO Regional Outlook (Revenue, USD Billion, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o Germany
o UK
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o China
o Japan
o India
o South Korea
o Australia
o Thailand
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa (MEA)
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the In vivo CRO Market Intelligence Study, published by Grand View Research.
Key Companies profiled:
• IQVIA Inc.
• Crown Bioscience
• Taconic Biosciences, Inc.
• PsychoGenics Inc.
• Evotec
• Janvier Labs
• Biocytogen Boston Corp
• GemPharmatech
• Charles River Laboratories
• Icon Plc
• Labcorp Drug Development
• Parexel International Corporation
• SMO Clinical Research (I) Pvt Ltd.
Recent Developments
• In November 2023, Charles River Laboratories announced the partnership with Aitia for drug development and in vivo oncology research.
• In March 2023, Biocytogen Boston Corp announced a licensing agreement with Janssen Biotech, Inc. that granted Janssen Biotech, Inc. the rights to use the RenLite platform.
• In January 2023, Evotec SE entered into a partnership agreement with Janssen Biotech to develop targeted immune-based therapies for oncology.