Creative Diagnostics, a leading manufacturer and supplier of antibodies, antigens and assay kits, is pleased to announce the availability of its new line of Norovirus VLP Antigens to accelerate in vitro diagnostics and vaccine research. These new antigens have been validated to a high level of confidence using electron microscopy.
Norovirus is a non-enveloped, positive-sense, single-stranded RNA virus of the family Caliciviridae and is the leading cause of non-bacterial acute gastroenteritis worldwide. The infection is characterized by non-bloody diarrhea, vomiting and abdominal pain. The stability of viral particles is of particular interest because noroviruses are transmitted by the fecal-oral route and they can persist in the environment. Studies of the assembly and degradation of norovirus capsids have relied heavily on norovirus-like particles.
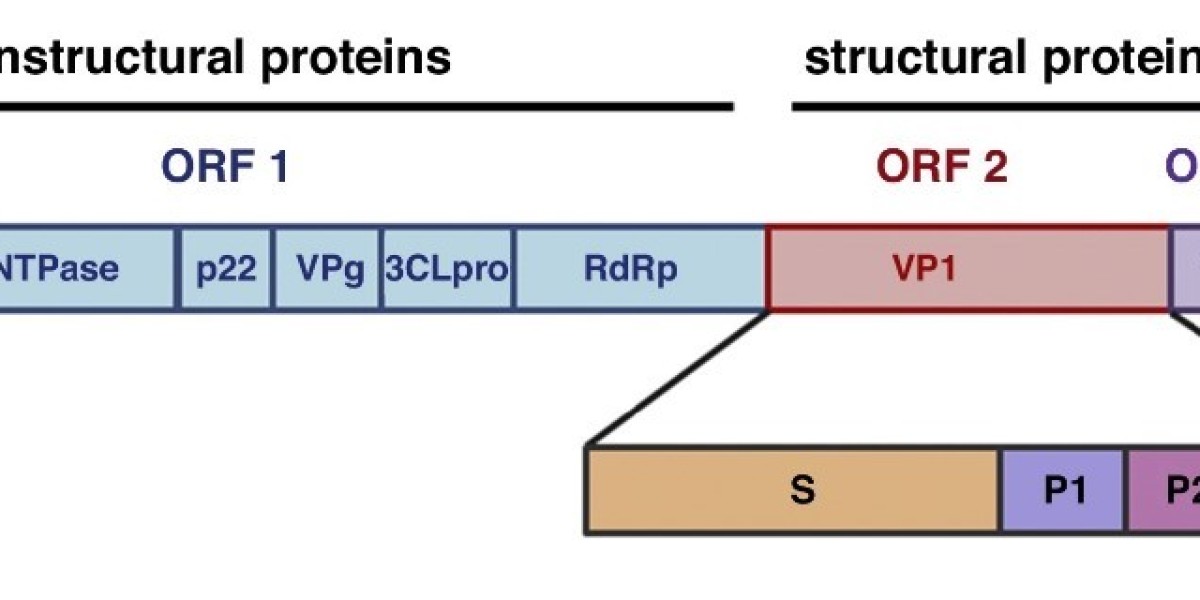
The norovirus genome is 7.4-7.7 kb in size and has three open reading frames (ORFs). Nonstructural proteins, such as RNA-dependent polymerase (RdRp), are encoded by ORF1, while structural proteins are encoded by ORF2 (VP1) and ORF3 (VP2). Structural analysis of noroviruses has shown that each viral capsule consists of 90 dimers of VP1 with T = 3 icosahedral symmetry. Various expression systems have been developed to produce viral capsids in the form of virus-like particles (VLPs).
Noroviruses are classified into genomic groups and genotypes based on the amino acid diversity of the VP1 protein. According to this system, the genus Norovirus is divided into 10 genomes (GI-GX) and 49 genotypes (9 GI, 27 GII, 3 GIII, 2 GIV, 2 GV, 2 GVI, and 1 each of GVII, GVIII, GIX [formerly GII.15 ], and GX), of which the genomes known to be infectious to humans include GI, GII, GVIII, and GIX. GII.4 is of particular importance as it has caused numerous outbreaks worldwide over the years and is believed to be the primary cause of human infections.
Creative Diagnostics now offers a series of yeast-expressed Norovirus VLP antigens for in vitro diagnostic and vaccine studies, including Recombinant Norovirus GI.1 VLP, Recombinant Norovirus GII.3 VLP, and Recombinant Norovirus GII.17 VLP. These antigens have been validated with high confidence by electron microscopy and provide researchers and diagnosticians with a powerful tool for studying and detecting Norovirus infections. Creative Diagnostics’ team of experts is also available to assist researchers in selecting the most suitable antigens for their specific studies.
The purified Norovirus VLP Antigens are an example of Creative Diagnostics’ commitment to providing innovative and reliable products to the research and diagnostic communities. Its team has worked diligently to develop antigens that are not only highly specific but also offer excellent sensitivity, making them ideal for a wide range of diagnostic and research applications.
Creative Diagnostics offers a comprehensive range of Norovirus VLP Antigens catering to diverse research needs. For more information on the new antigens, please visit https://www.creative-diagnostics.com/new-norovirus-vlp-antigens.htm.
About Creative Diagnostics
Creative Diagnostics is a leading manufacturer and supplier of antibodies, viral antigens, innovative diagnostic components, and critical assay reagents. In addition to providing contract RD and biologic manufacturing services for diagnostic manufacturers along with GMP biologics manufacturing for the biopharmaceutical market, the company aims to continue to act as a trusted source for all researchers’ assay development and manufacturing needs.